Beyond Li-ion batteries: performance, materials diversification, and sustainability.
Global recognition of the need to diversify energy storage in accordance with sustainability is driving the development of beyond Li-ion batteries.
However, the transition toward a truly sustainable energy industry necessitates informed cradle-to-cradle cost, performance, and environmental assessments together with introduction of long-term international legislation and concerted action from all stakeholders along the battery chain.
Batteries will play a significant role in reaching the global target of carbon neutrality by 2050. However, Li-ion batteries (LIBs), the current dominant technology, face increasing scrutiny over their dependence on critical materials such as Co and graphite, and their associated socio-environmental impacts.
🔥 What about we co-host a webinar? Let's educate, captivate, and convert the battery economy!
Batteries News is the global go-to online magazine for the battery industry, we can help you host impactful webinars that become a global reference on your topic and are an evergreen source of leads. Click here to request more details
Although LIBs will still be necessary for certain applications, the future energy landscape requires greater diversification of storage chemistries that can deliver higher energy, longer lifetimes, faster charging, and greater safety in an economical and sustainable manner.
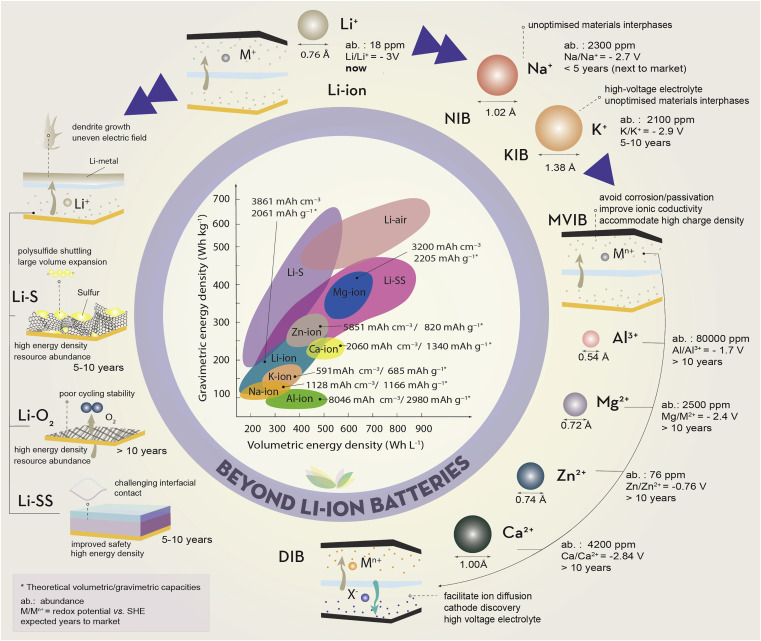
Extensive research has focused on alternatives to traditional cathode materials: for example, Li-S and Li-O2; all-solid-state configurations; substitution of Li for other alkali metals, Na and K; and multivalent-ion batteries (MVIBs) based on Mg, Ca, Al, or Zn1 (Figure 1).
Progress across these chemistries varies: MVIBs and Li-O2 are in the early stage of development, whereas the most advanced sodium-ion batteries (NIBs) are the focus of several manufacturers; indeed, CATL, Tesla’s primary battery supplier, intends to begin industrializing its technology on a large scale by 2023.
However, mainstream rollout of new batteries is hindered by both challenges specific to individual chemistry and wider universal factors.
Current status and challenges in developing beyond Li-ion technology
Battery chemistries beyond Li ion tend to either deploy metallic Li at the anode or substitute Li ions entirely, but both approaches face challenges. Li-metal anodes could allow access to energy densities an order of magnitude higher than current LIBs, but dendrite growth on the metal surface can lead to premature performance fade or, worse, explosive failure.
These safety issues can be addressed and energy density further improved by pairing Li-metal anodes with solid electrolytes (SEs) in Li solid-state (LiSS) batteries.2
However, SEs (typically ceramic- or polymer-based) can struggle to provide sufficient ionic conductivity, and in the case of ceramic electrolytes, many contain critical elements such as Ge, La, Zr, or Ti, although newer sustainable alternatives based on sulfides have emerged.
A LiSS configuration still typically employs conventional Li-ion cathodes, which are also dependent on critical elements, but these could be avoided with alternative cathode pairings such as Li-S and Li-O2.
However, although low-cost and abundant, sulfur cathodes suffer premature capacity loss from uncontrolled shuttling of soluble polysulfide intermediates,3 and Li-O2 batteries are compromised by a lack of cathode structures that allow constant flow of uncontaminated oxygen throughout cycling, with suitable catalysts to promote nucleation of Li2O2 over irreversible Li2O formation.4
Systems that substitute lithium with more abundant Earth elements such as Na and K operate analogously to LIBs, but incompatibility with particular Li electrodes has necessitated development of new, higher-capacity materials.1
Hard carbons have long been the anode of choice (up to 400 mAh g−1), but alloying materials such as Sn (847 mAh g−1) or Sb (660 mAh g−1) could offer potentially higher capacity, although effective methods of accommodating the large volume expansion must be developed to ensure electrode integrity.
Cathodes based on layered metal oxides, polyanions, or Prussian blue analogs enable the replacement of Co and Ni with non-critical redox species such as Mn or Fe, and switching from Cu to Al current collectors is expected to further reduce total cell cost.
However, energy densities are limited by the larger ionic radius of Na and K ions, limiting uptake to applications where battery size is less critical.Multivalent-ion chemistries could be attractive for EVs and portable electronics by offering specific and volumetric energy densities beyond current state of the art (Figure 1).
However, higher charge density on the mobile ion will likely complicate their practical application. The ability of metal oxides, chevrel, and spinel structures5 to facilitate fast ion diffusion has been explored, but discovery of cathodes able to accommodate highly polarizing ions, and electrolytes that avoid anode passivation and corrosion, with sufficient ionic conductivity, remains challenging.
Provided suitable cathodes and electrolytes that are stable under high voltages can be found, one promising avenue of research that could allow for high power is the combination of metal-ion intercalation at the anode with counterion intercalation at the cathode in a dual-ion battery configuration.6
For many of these chemistries it will be possible to employ the same processes with only slight modifications. Indeed, some steps might be simplified: employing Li-metal anodes precludes the need for dispersing, coating, or calendering traditional electrodes.
Conversely, the greater moisture sensitivity of sodium cathodes requires more stringent manufacturing conditions, alongside discovery of cathodes that can withstand aqueous processing. The few sustainability and economic assessments that exist for new technologies (mostly NIBs) indicate socio-environmental and economic cost reductions because the manufacturing costs are assumed to be similar.7
However, although switching to new chemistries alleviates pressure on certain material demands, it inevitably introduces new ones; for example, although Li-S and Li-O2 batteries substitute abundant elements at the cathode, they continue to rely on lithium for the anode.
Furthermore, a universal problem facing all chemistries is that battery production still has a significant greenhouse gas footprint that must be addressed, and comprehensive sustainability evaluation is complicated by insufficient long-term performance data and the lack of harmonization across life cycle assessment (LCA) methods.
Lastly, we face a large-scale problem of waste disposal as first-generation LIBs reach their end of life. Efforts are growing to develop effective recycling methods, but variations in battery chemistry and difficulty in separating packs into their constituent components complicate recycling practices.
Furthermore, current processes only recover high-value materials such as Co, and recycling is thus highly resource inefficient and economically unfavorable.
Measures to enable full-spectrum sustainability
At present, no single emerging battery chemistry can match LIBs on every performance point, but future innovations must think beyond performance and consider how to reconcile technological advances with the economic and environmental implications associated with each step of the battery value chain.

Develop high-throughput, centralized testing
Battery performance is governed by additional factors beyond the storage capacities of the cathode/anode. Electrode structure, electrolyte and binder properties, and any other components within the cell play a role.
Typically, rather than adopting a whole-cell perspective, the scope of individual academic research may be limited to only one particular aspect of battery chemistry.
Developing a centralized and open-access high-throughput combinatorial testing facility will allow the convergence of individual materials discoveries from across the academic community to easily and efficiently discover viable new battery chemistries and configurations.
The application of machine learning to the large datasets generated from this high-throughput automated process, which is emerging in other areas including photocatalysis and photovoltaics, will further accelerate discovery and development of new components by determining optimized target materials and combinations for subsequent synthesis and testing.
As outlined in the EU Big-Map Battery 2030+ project,8 adopting a centralized approach will be key to bringing together research outcomes from across the breadth of battery research and will overcome the main challenge facing machine learning: an insufficiency of consistent and reliable data.
In parallel to materials innovation, design of advanced characterization techniques must be pursued to allow comprehensive operando characterization of cells undergoing cycling to better study chemical and morphological phenomena occurring within the electrodes and interfaces.
Design in advanced multifunctionality
The development of advanced multifunctionality will help address the challenge of battery lifetime; for example, introducing self-healing formulations that respond to mechanical or chemical stimuli could preserve the structural integrity of the electrode, thus preventing premature degradation and failure.9
Meanwhile, implementing in situ non-destructive health diagnostics will allow users to better understand the battery’s lifespan and facilitate safer and more efficient use conditions.
At the pack scale, determining optimal cycling conditions for each chemistry and application will ensure that users extract the optimum performance-lifetime balance.
Innovations in modular designs should also be prioritized to allow for disassembly of spent batteries, for example, by employing compression to reduce the need for adhesives and to facilitate dismantling (e.g., Aceleron Energy, UK).
Design for end of life
Battery recycling must become routine. Implementing measures such as full traceability of material composition with accountability at each stage of manufacturing will require compliance from manufacturers and policymakers.
Design for either full biodegradability or disassembly should be paramount, with consideration of engineered surfaces for easy component disassembly, while the use of water-soluble binders will facilitate separation.
Where pyrometallurgical processes cannot be avoided, development of less-energy-intense treatments should be pursued, such as low-temperature smelting, as developed by Umicore. Integrating a high degree of automation will result in greater efficiency, which will be significantly simplified by manufacturing standardization.
Comprehensive cost or energy analyses for beyond-Li recycling are scant, due to insufficient end-of-life batteries to study. Nonetheless, as the LIB recycling industry matures, it is anticipated that recycling techniques for spent LIBs could be extrapolated to NIBs, which share significant cell chemistry with LIBs.
Although materials criticality is less important for future battery technologies, more holistic sustainable practices should be tailored into the manufacturing of battery packs from the outset, rather than considered retrospectively.
Whether a one-size-fits-all recycling approach will be possible or whether it will be necessary to tune recycling technology to each specific chemistry remains to be seen, but certainly the added cost of establishing flexibility will require an initial financial incentive to encourage widespread adoption.
Target abundant materials and enforce stricter mining and refining practices
The cost of raw materials, including Li, Co, Ni, and Mn, raises the cost of batteries.10 Mining of these strategic metals is also inseparable from environmental pollution and social/ethical issues.
Alternative cathodes that avoid Co, and chemistries based on abundant and globally evenly distributed raw materials such as Na and K, will reduce the financial, environmental, and social burdens.
However, these replacements will likely still rely heavily on primary rather than secondary resources, and, thus, less exploitative and energy-intensive extraction processes must be developed.
Ensure a resilient local supply and manufacture chain
Securing a local supply chain would reduce raw materials cost and enhance the resilience of supply while supporting local populations. As technologies mature, local recovery from recycled batteries could further ensure security of supply and help close the loop to develop a circular economy.
Reduce energy demands of manufacturing and switch to greener processes
New battery chemistries could enable positive changes to manufacturing practice. Carbon materials can be found across all chemistries as either active material or conductive additive; thus, switching from petrochemical to biomass precursors will alleviate fossil dependence.
Using biomass-derived aqueous binders, such as carboxymethyl cellulose, or lowering pyrolysis temperatures will reduce the energetic and economic costs of battery production.
Where energy-intensive processes cannot be circumvented, clean electricity sources should be prioritized. Synthesis and processing with harmful solvents should also be minimized, with the aim of full recovery if unavoidable.
Implement clearer labeling and accountability
The EU proposes a unified battery passport11 containing information including cell chemistry, origin, first application, state of health after first life, and chain of custody. Third-party valuation platforms to assess state of health will assist in the exchange of used batteries.
Clear and transparent labeling at all stages of the battery’s life must also clarify the extended responsibilities of battery producers: for example, how liability is transferred when a battery is refurbished for a second-life application.
Concerted cooperation, policy agreement, stronger legislation, and financial incentives
The success of the coming law outlined in the battery passport initiative11 to establish a sustainable battery future requires concerted cooperation from all stakeholders, including manufacturers, investors, policymakers, and international regulatory bodies, at all stages of the value chain as well as greater transparency and better harmonization across the industry (Figure 2).
Open-access and reliable LCA tools will enable consistent and comprehensive sustainability evaluation. In the near term, several of our proposed measures are perhaps economically unfavorable and will require significant policy agreement or financial encouragement.
The removal of fossil fuel subsidies and development of instruments that incentivize the adoption of renewable products throughout the value chain would be beneficial.Stronger regulations and financial incentives will most benefit the recycling of spent batteries; harvesting end-of-life value from batteries could reduce greenhouse gas intensity by 34 Mt and create economic value of ∼$35 billion.12
Recycling and recovery of current LIBs could provide a local and steady source of Li, alleviating pressure on virgin extraction, while Co and Ni can be recovered for cathode use.
However, current recycling economics are only favorable due to the high recovery price of Co, so until sufficient economies of scale are reached, financial and regulatory stimuli will likely be necessary to incentivize the recycling of lower-value materials employed in new chemistries.
A strong precedent in this regard is the near 100% recovery and recycling of lead-acid batteries despite their far lower value in comparison with that of LIBs.13 Meanwhile, aluminium’s established recycling infrastructure allows 35% of Al demand to be supplied through secondary Al,14 despite high Earth abundance.
The main driving force for battery development has been performance and economics, but at the expense of sustainability. A more inclusive long-term vision is required to enable a supply chain based on full-spectrum sustainability that fosters a transition toward a circular economy; such an approach will decrease the environmental, social, and economic footprint of batteries.
The recommended practices outlined here will enable the residual value of used batteries to be fully extracted. Harvesting of their end-of-life value will also necessitate local workforces and therefore create new job opportunities.
In time, this future shift will likely come naturally as the end-of-life battery market grows, and the high volume of recyclable materials or components enables this industry to become profitable, but time is not on our side, and, thus, enacting legislation and economic reliefs now will be crucial to driving these changes.
READ the latest Batteries News shaping the battery market
Beyond Li-ion batteries: performance, materials diversification, and sustainability, March 18, 2022








2 comments